The Background
Endoscopy provision across West Yorkshire and Harrogate, as nationally, has been significantly impacted by the pandemic, resulting in a backlog of patients.The impact of social distancing and additional protective measures mean that this situation is likely to continue for the foreseeable future. This has meant that many patients who would normally be followed up for Barrett’s oesophagus or investigated for cancer using traditional endoscopy have had to wait longer.
More information about endoscopy
What is Cytosponge?
Cytosponge is a new innovative test which was developed to identify Barrett’s oesophagus – a condition that can increase a person’s risk of developing oesophageal (food pipe) cancer. It’s an inexpensive and simple test that can be done in a GP surgery or hospital setting outside of the traditional endoscopy suite. Early trials showed that it was safe and acceptable to patients.
Cytosponge is a soluble capsule that contains a small sponge or a ‘sponge on a string’. The patient swallows the capsule which has a thread attached. A small sponge is release from the capsule and a trained nurse/clinician pulls on the thread to withdraw the sponge. As the sponge comes back up the gastrointestinal tract it collects small samples of cells that can then be sent to pathology for analysis.
Partners In The Project
- West Yorkshire & Harrogate Cancer Alliance
- Six acute Trusts in West Yorkshire and Harrogate (West Yorkshire Association of Acute Trusts)
- University of Cambridge
Benefits
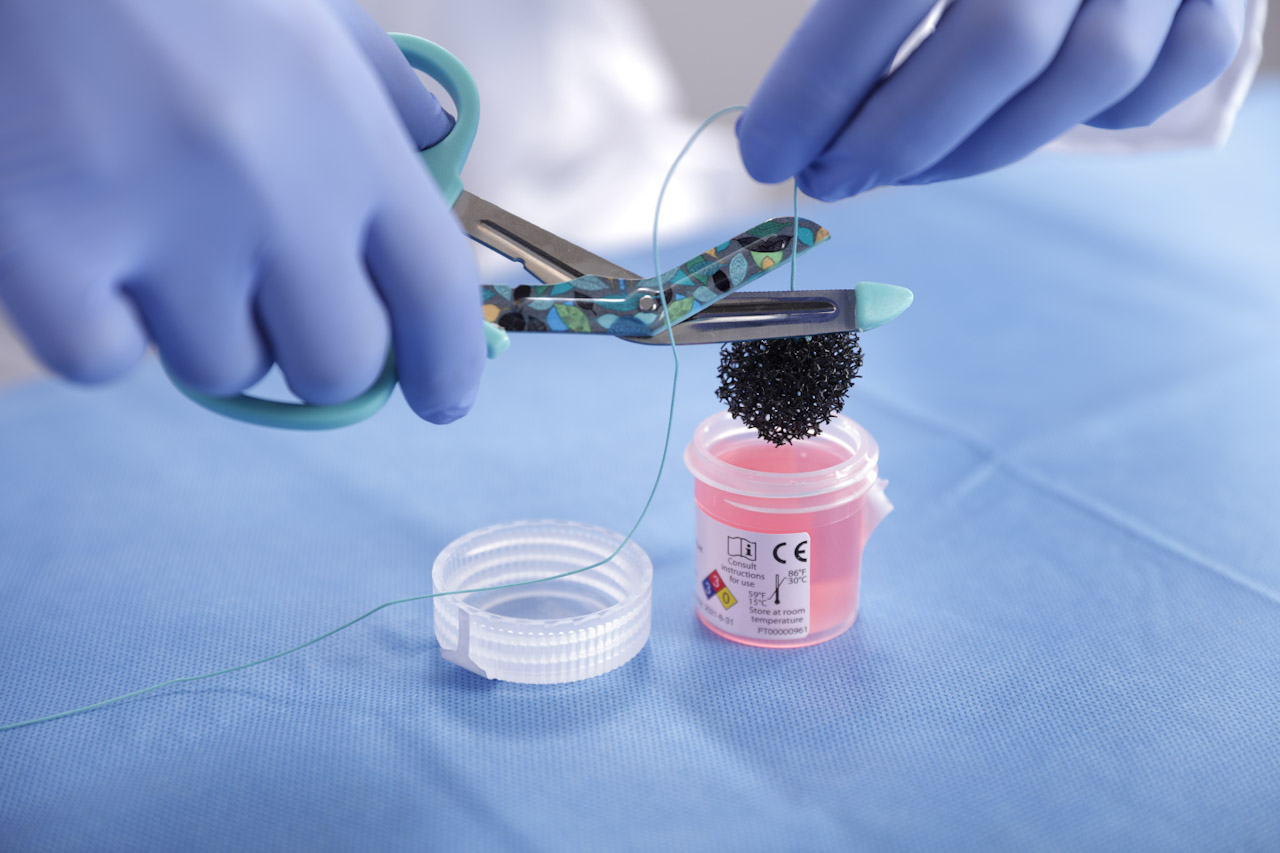
One major benefit in the recovery period from COVID19 is that the procedure is minimally-aerosol generating reducing the risk to the patient and healthcare professional alike. It can be delivered outside an endoscopy suite in a separate clinical room, including in the longer term in Primary Care. There are benefits to the patient in terms the ease of the procedure in comparison to Endoscopy and that it can be delivered, for some patients at an accessible venue outside the hospital environment.
Picture, right, courtesy of Medtronic.
Why offer the test now?
A randomised trial showed that offering Cytosponge test to individuals on medication of heartburn increased the number of cases of Barrett’s diagnosed by 10-fold and this also included the identification of some early cancers. Following these initial trials, additional research is being rolled out to understand its effectiveness in investigating upper gastro-intestinal symptoms that might be cancer and to identify those most in need of urgent treatment.
During COVID since routine endoscopy including for screening and surveillance has been curtailed. Initially Trusts across West Yorkshire and Harrogate will be joining a trial of Cytosponge for patients with known Barrett’s who would usually have endoscopic surveillance of their condition which can develop into Cancer. The outcome from the Cytosponge will help to prioritise patients who need to then be referred to endoscopy
Read more:
Cancer Research UK Science Blog Case Study - A Sponge On A String To Detect Oesophageal Cancer Earlier
Heartburn Cancer UK News - University of Cambridge project aiming to improve early diagnosis of oesophageal cancer receives a share of £16m funding from UKRI









