Innovations Programme
The West Yorkshire and Harrogate Cancer Alliance has joined forces with innovators and clinicians to support the introduction of new technology and new ways of working which will help to support the delivery of cancer services during the Covid pandemic and beyond.
There is a particular focus on improving early diagnosis through risk assessment and new diagnostic techniques.
PinPoint, a new blood test for patients with cancer symptoms, will help clinical staff identify patients with the highest risk of having cancer, ensuring those patients have their diagnostic tests as soon as possible.
Cytopsonge and Colon Capsule Endoscopy will provide alternatives to endoscopy, an investigation using a camera at the end of a tube to examine the body internally. Traditional endoscopy procedures were all paused during the early days of the pandemic due to the use of air which helps the camera move though the patient’s body. Speedy introduction of alternatives would therefore be of great benefit now and in the future.
Picture, right, courtesy of Medtronic
Links With The National Team
The newly formed West Yorkshire and Harrogate Cancer Alliance Innovation Team is linking with the National Innovation Programme to bring nationally sponsored innovation projects into the area. The national team was established in 2020/21 with the aim of enabling:
- Testing and evaluation of prioritised innovations in a ‘real world’ setting
- Accelerated roll-out of prioritised innovations
and to support the Long Term Plan cancer ambitions:
- Diagnose 75% of cancers at stages one and two
- An extra 55,000 people each year will survive for five years or more following their cancer diagnosis
- Speed up the path from innovation to business-as-usual, spreading proven new techniques and technologies and reducing variation
Here is a summary of the West Yorkshire and Harrogate Cancer Alliance innovation projects:
The PinPoint Test
The PinPoint Test is a newly developed decision support tool for clinicians, which produces a calibrated probability of a patient having cancer in a given urgent referral/two week wait pathway. The test uses machine learning to combine results from multiple blood analytes with basic patient information. This will allow clinicians to easily identify those patients who should be further investigated by specialists for cancer diagnosis, red flagging particularly urgent cases and identifying those who can be safely investigated for other possible causes of their symptoms. The clinician will make their decision on prioritising the patient by looking at both the patient’s symptoms and the PinPoint Test result.
As part of a formal service evaluation, the test will run in parallel to existing services until 100 cancer diagnoses per cancer site have been confirmed. This evaluation will then be signed off by an expert NHS panel before the test results can be used by clinicians. The service evaluation will be rolled out in September 2020 to GP practices in Wakefield and North Kirklees, with other areas following as rapidly as possible.
As part of Pinpoint’s further spread across West Yorkshire and Harrogate, Pinpoint was rolled out to Leeds Breast Clinics in May 2021 to help not only secure further tests within a faster timeframe but to also ensure that the test reaches the threshold of over 100 cancer diagnoses so it can then become part of patient care. PinPoint will be rolled out to include patients being referred with other cancers during 2021.
Learn more about the PinPoint Test Graphic images courtesy of Ink - www.designedbyink.co.uk
Cytosponge
 West Yorkshire and Harrogate Cancer Alliance has joined a large trial to bring cytosponge in to use in standard clinical settings.
West Yorkshire and Harrogate Cancer Alliance has joined a large trial to bring cytosponge in to use in standard clinical settings.
Project DELTA, is funded by Innovate UK and led by Professor Rebecca Fitzgerald and her Cambridge research team. It aims to improve the diagnosis of oesophageal cancer. It is a collaboration between the Universities of Cambridge, Oxford, King's College London, the PHG Foundation and Cyted. Advisory Board support is provided by Action Against Heartburn, Heartburn Cancer UK, the West Yorkshire and Harrogate Cancer Alliance and Newcastle University.
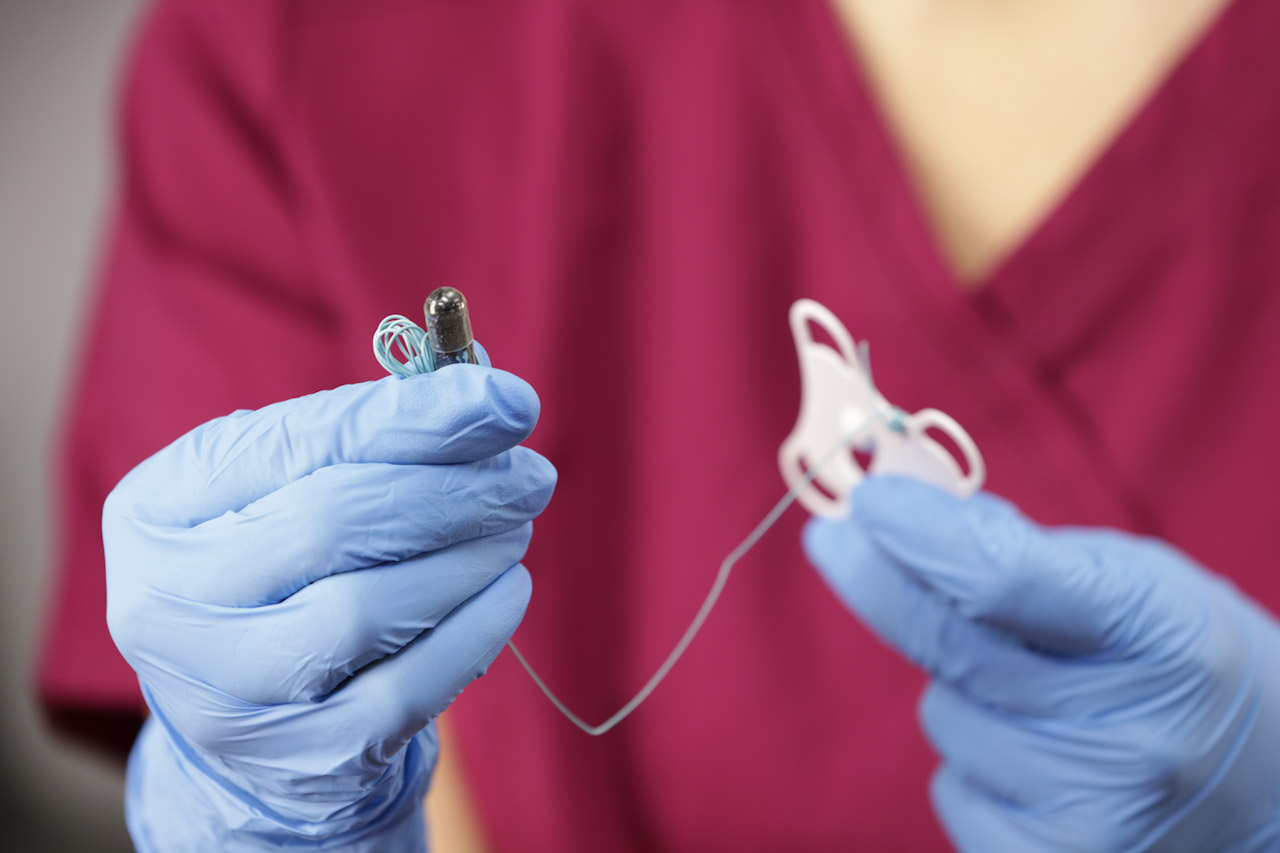
Picture, left, courtesy of Medtronic.
Visit the Cancer Alliance Cytosponge web page
Visit the Project Delta website
Cytosponge is a new device which can be used to investigate upper gastro intestinal symptoms that might be cancer and to identify those most in need of urgent treatment. This innovative test that has been developed to identify Barrett’s Oesophagus – a condition that can increase a person’s risk of developing oesophageal (food pipe) cancer. During previous research trials, this test found a higher than expected number of early adenocarcinomas. It’s an inexpensive and simple test that can be done in clinic and for some patients, can replace the need for an endoscopy.
The cytosponge is a capsule that contains a small sponge. The patient swallows the capsule, which has a thread attached. A small sponge is release from the capsule and a trained nurse/clinician pulls on the thread to withdraw the sponge. As the sponge comes back up the gastrointestinal tract, it collects small samples of cells that can then be sent to pathology for analysis.
New: National Cytosponge Programme - West Yorkshire and Harrogate Cancer Alliance has volunteered to join the first phase of an initial pilot - linked to but not part of the Cambridge programme - in quarter 4 of 2020/21. This will continue into 2021/22.
Colon Capsule Endoscopy
Colon Capsule Endoscopy is a new procedure which offers an alternative to colonoscopy for some patients.
The procedure involves the patient swallowing a small capsule which takes pictures of the inside of the colon as it passes through the patient. Images are sent to a receiver that the patient wears on a belt. The pictures recorded are then analysed by a trained Nurse and Clinician to identify any abnormalities.

NHS England is supporting the introduction of Colon Capsule Endoscopy as part of their COVID recovery response. This project will focus on patients with a positive FIT Test of 10mg to 100mg. The Cancer Alliance will be supporting implementation in West Yorkshire and Harrogate.
West Yorkshire & Harrogate clinicians are interested in exploring the use of capsule endoscopy for lower risk patients, with a FIT test of less than 10mg, who may otherwise need to wait longer for investigations. 1% or patients in the lower risk categories are known to have cancer and approximately 16 % will have polyps and may require further management. Picture, right, courtesy of Medtronic.
This project will be funded by the Cancer Alliance for implementation by local acute Trusts.
Endoscopy guidance from NHS England - Clinical guide for triaging patients with lower gastrointestinal symptoms 16 June 2020, Version 1
More information about Colon Capsule Endoscopy
FIT (Faecal Immunochemical Testing) For High Risk Patients
Faecal Immunochemical Testing (FIT) is a process that is already used as part of the Bowel Cancer Screening Programme to identify the likelihood that a patient has lower gastro-intestinal cancer and as a ‘rule out’ decision support tool for patients who present to their GP with symptoms, but don’t meet the criteria for an urgent cancer referral.
As part of the response to COVID, the test is being extended to patients who have been referred with suspected lower gastro intestinal cancer symptoms. In this situation the FIT test will be used to enable the clinical team to assess the risk that a patient has cancer and to fast track high risk patients and look at different approaches to the management of low risk patients.
This work is being taken forward through the Cancer Alliance colorectal Optimal Pathway Group.








